IU/JAX/PITT Preclinical Testing Core
Stacey J. Sukoff Rizzo, PhD, Co-Director
Paul R. Territo, PhD, Co-Director
ABOUT THE PRECLINICAL TESTING CORE
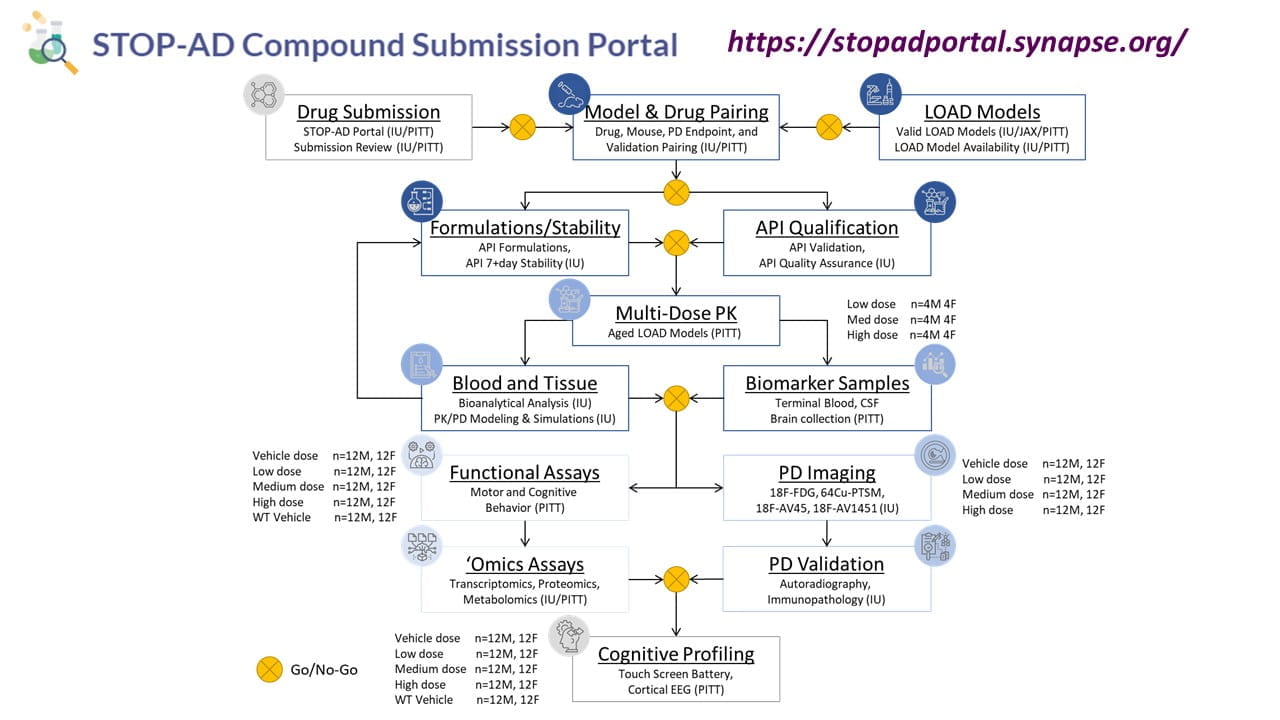
The PTC is a group of researchers under the direction of Drs. Paul Territo at Indiana University (IU) and Stacey Rizzo at the University of Pittsburgh (PITT).
The PTC has established a streamlined preclinical strategy for compound screening with go/no-go decision points that allow critical and unbiased assessments of potential therapeutic agents. The PTC strategy includes a primary screen to determine: 1) drug formulation; 2) drug stability; and 3) in vivo pharmacokinetics and target tissue concentrations in mouse models characterized by MODEL-AD at pathophysiologically relevant ages. A secondary screen evaluates target engagement and disease modifying activity utilizing non-invasive PET/MRI as a pharmacodynamic (PD) readout matched to known disease pathology in the model. Compounds demonstrating positive PD effects in the secondary screen are further interrogated via a tertiary screen of functional assays that assess the compound’s ability to normalize a disease-related phenotype in cognition and neurophysiological tests. The PTC provides resources for drug screening to the greater AD research community through the STOP-AD portal at minimal costs to the investigator. For more information on drug screening resources, please see the STOP-AD Compound Submission Portal.